| Femtosecond X-ray Studies of Solution-phase Photochemistry – Robert Schoenlein |
Atomic and Electronic Structural Dynamics in Transition-Metal Spin-Crossover Complexes
Understanding the ultrafast structural dynamics of photo-induced phase transitions and chemical reactions in molecular complexes is an important scientific objective, both for developing new materials and devices, and for understanding fundamental chemical and biological phenomena. Of particular interest are transition-metal spin-crossover complexes in which the d-electronic energy structure is strongly modified by the ligand field. Photo-excitation to the charge-transfer (MLCT) state gives rise to a spin interconversion (ΔS=2) on a remarkably fast (sub-picosecond) time scale – raising important questions about the role of dynamic changes in the ligand structure (ligand field) in facilitating this transition. We address these questions for the first time using time-resolved x-ray spectroscopy to provide quantitative information on the atomic and electronic structural dynamics.
Structural Details FeII Photoexcited High-Spin State at 330 ps
Synchrotron-based picosecond XAS provides the first direct insight into the atomic structural changes associated with the spin crossover transition of an Fe(II) complex in solution with 0.03 Å spatial and 70 ps temporal resolution. Our results show that within our time resolution a dilation of the Fe–N bond length (by 0.2 Å) is a good reaction coordinate for describing this prototypical intramolecular electron transfer reaction in solution. This experiment paves the way for future femtosecond x-ray absorption studies of solvated molecular complexes at the Advanced Light Source.
Solvated Transition-metal Complexes
Charge-transfer processes in transition-metal complexes are of fundamental interest due to the strong interaction between electronic and molecular structure. In particular, FeII complexes ADDIN ENRfu exhibit strong coupling between structural dynamics, charge-transfer, and spin-state interconversion. The spin-crossover transition is closely related to the electron transfer reactions in heme proteins and is of practical interest for opto-magnetic storage via light-induced excited spin-state trapping (LIESST) ADDIN ENRfu .
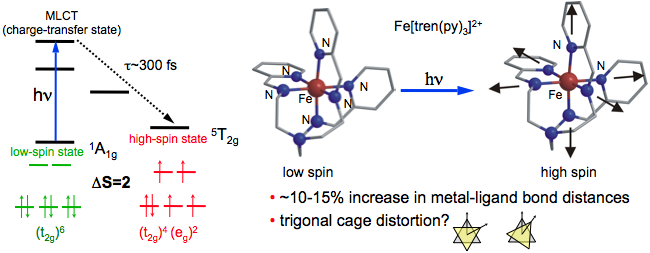
The FeII complex consists of an Fe atom with octahedrally coordinated ligands characterized by six nearest neighbor N atoms. Optical excitation from the low-spin state (1A11) to the metal-to-ligand charge-transfer (1MLCT) state initiates a rapid spin-crossover to the high-spin state (55T2). The speed (~300 fs) and efficiency of the spin transition (ΔS=2) suggest that the initial wavepacket dynamics play an important role in determining the reaction pathway. Changes in the Fe-N bond lengths may facilitate the spin interconversion by modifying the spin-orbit coupling. The scientific motivation for time-resolved X‑ray studies is to understand the role of structural dynamics by directly monitoring changes in the Fe-N bond distances during the reaction via time-resolved EXAFS at the Fe K-edge.
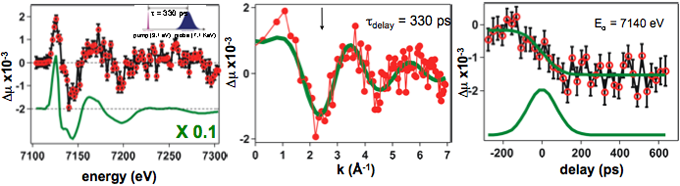
We performed the first direct measurements of the dynamic atomic and electronic structural rearrangements occurring during a photoinduced FeII spin crossover reaction in solution via picosecond X‑ray absorption spectroscopy. 100 fs pump pulses at 400 nm initiate a charge-transfer transition in the low spin complex. Subsequent electronic and geometric changes associated with the formation of the high-spin excited state are probed at the Fe K-edge (7.1 keV) with 70 ps, tunable X‑ray pulses. Modeling of the transient XAS data reveals that the average iron–nitrogen (Fe–N) bond is lengthened by 0.21±0.03 Å in the photo-excited high-spin state relative to the ground state within 70 ps. This structural modification causes a change in the metal-ligand interactions as reflected by the altered density of states of the unoccupied metal orbitals.
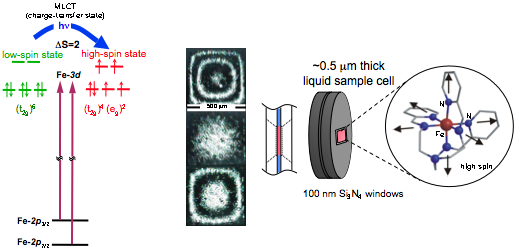
The electronic structural dynamics of the FeII spin transition are revealed via time-resolved XANES at the Fe L-edge (707 eV). L-edge X‑ray absorption spectroscopy directly probes the ligand-field split Fe-3d levels from the spin-orbit split Fe-2p levels. Thus, these selection rules can be exploited to extract quantitative information on the dynamics of the electronic structure; particularly the spin state of the system, Fe oxidation state, and the strength of the ligand-field parameter (10Dq).
A significant advance for liquid-phase experiments in the soft X‑ray range has been the development of a nanofluidic cell, suitable for use in vacuum, with controllable thickness in the 1 μm range for transmission measurements. Fabry-Perot interference fringes from a cell indicate thickness control on the sub-micron level.
Transition-metal Carbonyls
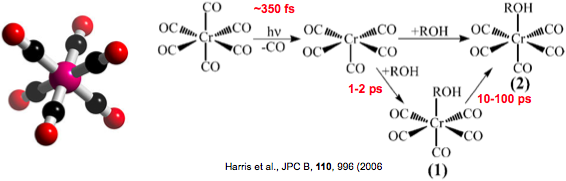
Figure: Reaction dynamics of transition-metal carbonyl, Cr(CO)6 in alcohol. Initial photodissociation occurs within 400 fs, followed by solvent complexation and ligand rearrangement.
Transition-metal carbonyls are a prototypical model system for understanding solution-phase photodissociation and ligand-exchange reactions in solution. In the gas phase, photoexcitation leads to detachment of multiple CO ligands whereas photolysis of solvated metal-carbonyls leads to the detachment of a single CO ligand followed by solvent complexation and ligand rearrangement. This is a direct consequence of enhanced energy relaxation, dissipation, and caging from the solvent environment. Thus, intermolecular interactions with the solvent bath have a strong influence on the intramolecular dynamics, and simple potential energy surfaces are generally inadequate to describe such condensed-phase reactions.
Much of the present understanding of solvated metal-carbonyls dynamics comes from femtosecond visible spectroscopy and is based on associating kinetics (time constants) with appropriate molecular processes. Extracting quantitative information about the reaction pathway and clearly identifying intermediate molecular structures from transient visible absorption spectra remains a significant challenge.
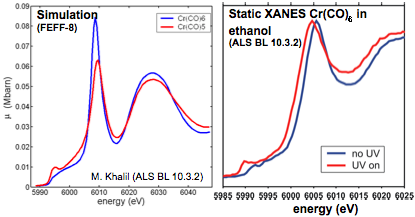
Time-resolved XAS will provide a more quantitative understanding of transition state structures, ligand substitution and rearrangement dynamics, and the role of the solvent in metal carbonyls. In the near-edge region, time-resolved XANES at the Cr K-edge follows the transient changes in the oxidation state of the transition metal following femtosecond photoexcitation and may enable the identification of transient intermediate species (e.g. D3h3h and C4v isomers) that are predicted during the initial relaxation of the Cr(CO)5 fragment.
Time-resolved EXAFS will directly measure changes in the Cr-CO bond distance during dissociation. Such measurements will also be sensitive to changes in coordination of the transition-metal associated with geminate recombination, ligand substitution, and subsequent rearrangement dynamics. An important question to be addressed is the influence of the solvent (e.g. viscosity, chain length, polarity) on the reaction dynamics.
Structural Dynamics of solvated halogen molecules and complexes
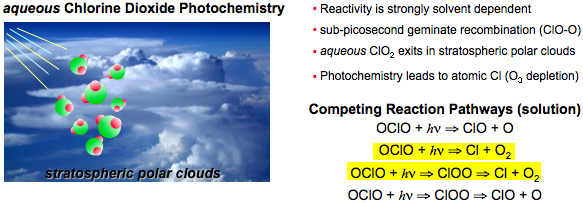
Time-resolved X‑ray techniques will provide important new information on the photo-induced reaction dynamics of aqueous chlorine dioxide (O-Cl-O). Solvated ClO22 is present in stratospheric polar clouds and plays a significant role in sunlight-induced atmospheric chemistry due to its ability to produce atomic Cl. A number of groups have applied femtosecond optical techniques to understand the pathways and the dynamics of this reaction, and there is evidence of geminate recombination occurring within 1 ps. However, optical measurements are unable to clearly identify intermediate species, or distinguish between the competing reaction pathways that appear to be solvent dependent.
We can achieve a clearer understanding of OClO photodissociation by using time-resolved EXAFS and XANES to measure the time-scale of the reaction dynamics (following femtosecond excitation in the near UV, ~400 nm), and to distinguish between the various reactant species during the course of the reaction. EXAFS measurements at the Cl K-edge monitors changes in the Cl coordination while XANES measurements can distinguish between OClO, ClOO, and Cl via distinct absorption features which reflect the various oxidation states.