Overview
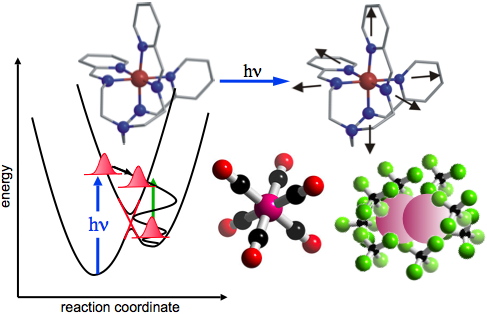
A fundamental challenge in condensed-phase chemistry is to understand the dynamic interplay between electronic structure (energy levels, charge distributions, bonding, spin) and molecular structure (atomic arrangement, bond distances, coordination etc.). The “transition state” intermediate between reactant and product species is of critical significance since subtle conformational changes and chemical bond formation/dissolution in this regime ultimately determines which reactive pathways are favored.
The central goal of this research program is to apply ultrafast X‑rays to develop new insight into condensed-phase molecular dynamics. Time-resolved measurements of XANES (X‑ray Absorption Near Edge Structure) can provide detailed information about dynamics of the valence charge structure, while time-dependent EXAFS (Extended X‑ray Absorption Fine Structure) provides information about changes in the local atomic structure. X‑ray measurements on the time scale of a vibrational period provide important new information about solution-phase chemical reactions including: conformational changes, formation/dissolution of bonds, charge transfer, changes in oxidation state etc.
Three areas of present focus are (1) solvated transition-metal complexes exhibiting strong coupling between molecular structure and electronic properties arising from the ligand field, (2) small halogen molecules exhibiting solution-phase reactivity that is markedly different from that in the gas phase, and (3) structural dynamics in liquid water under direct vibrational excitation. In the future we hope to extend this approach to molecular crystals exhibiting novel cooperative effects.